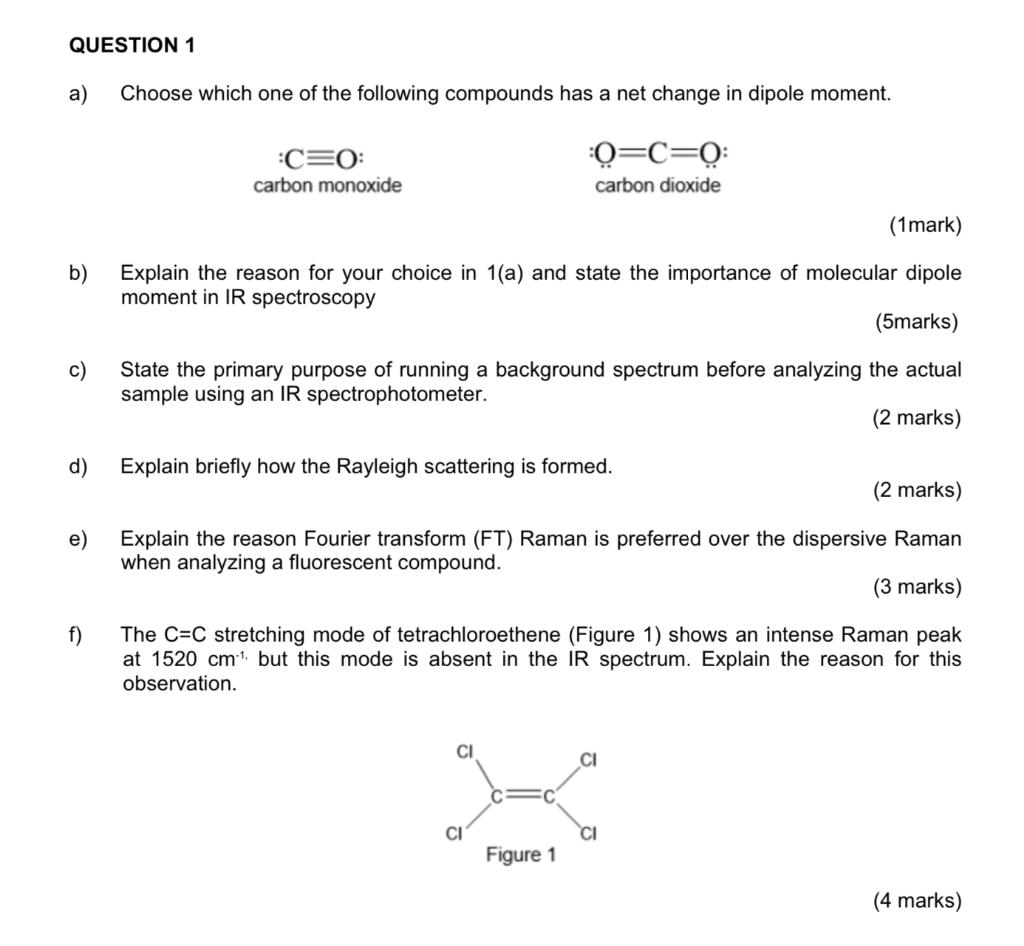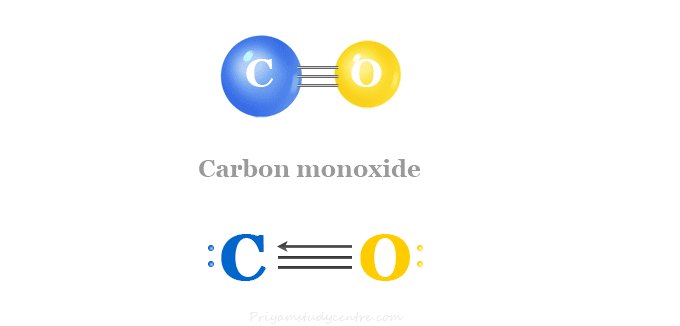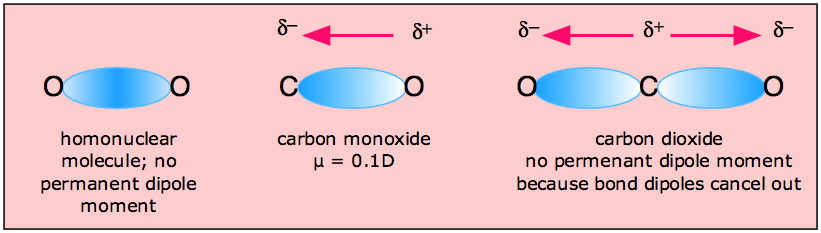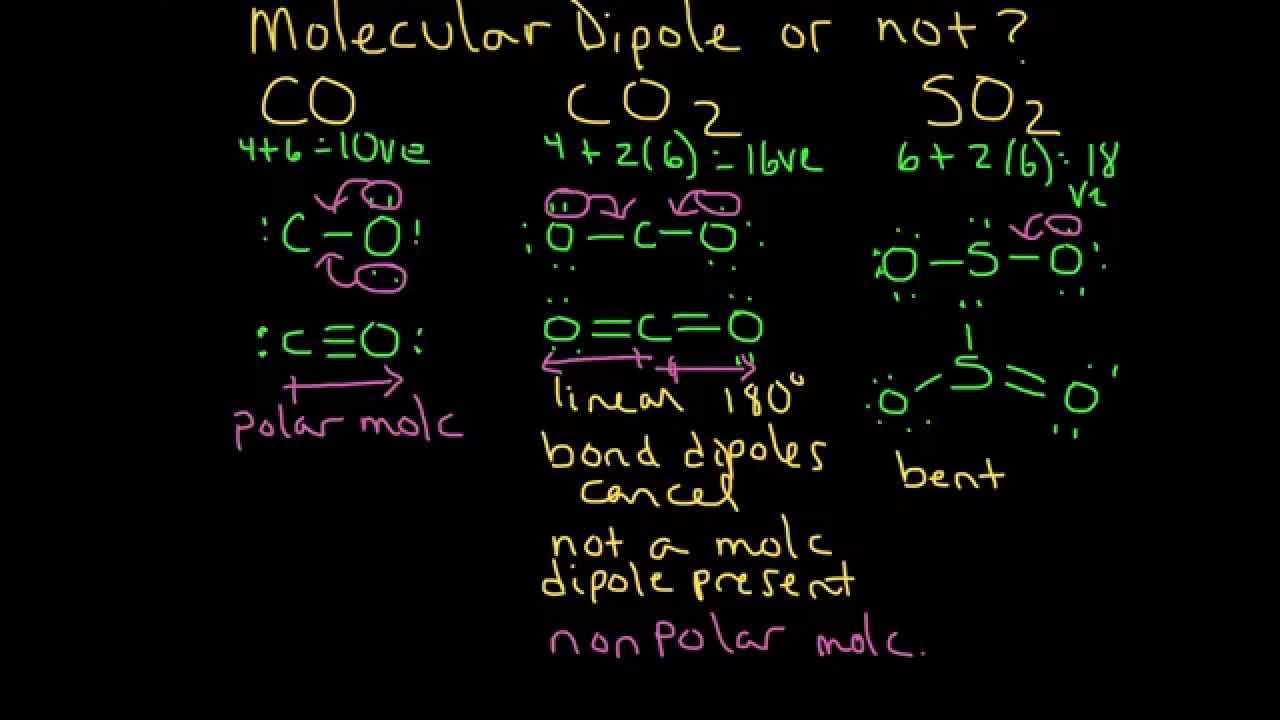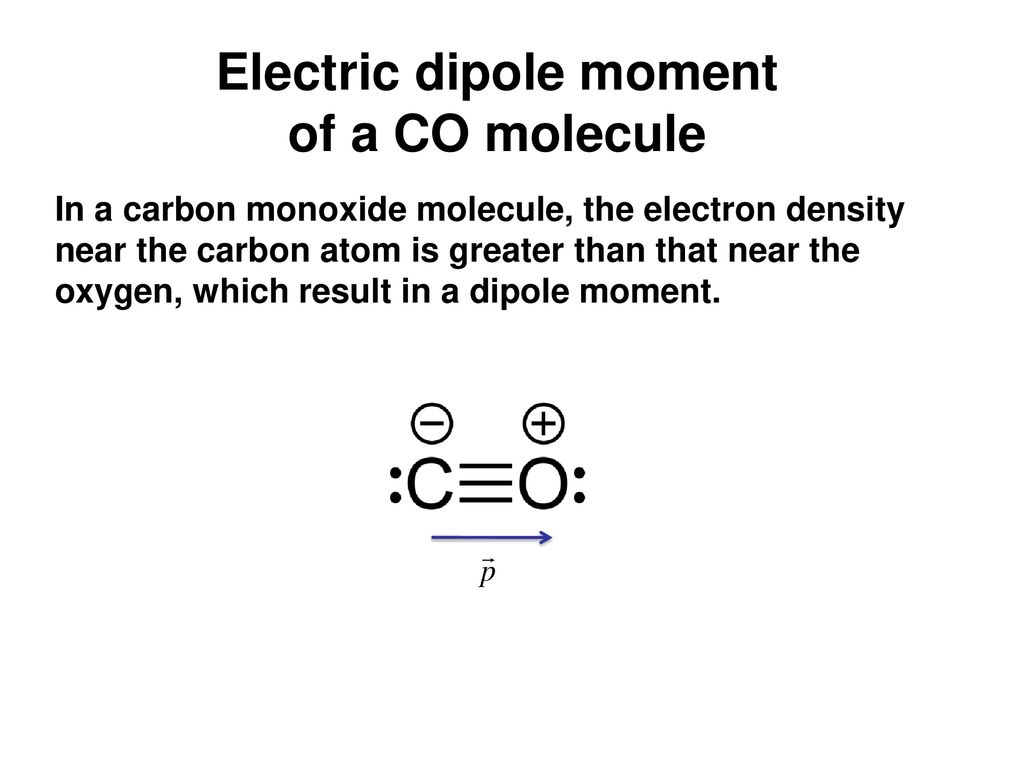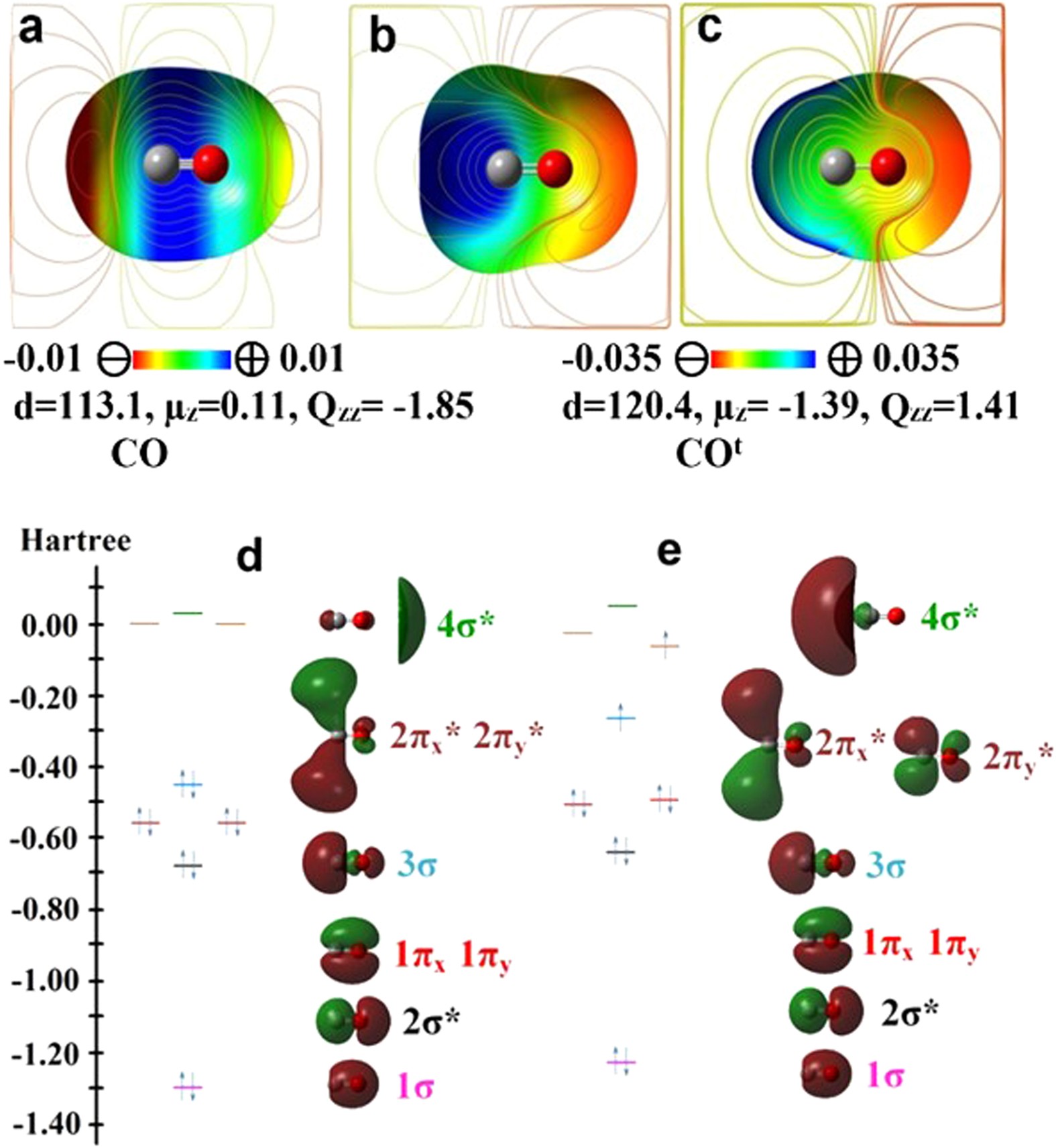
Intriguing Electrostatic Potential of CO: Negative Bond-ends and Positive Bond-cylindrical-surface | Scientific Reports
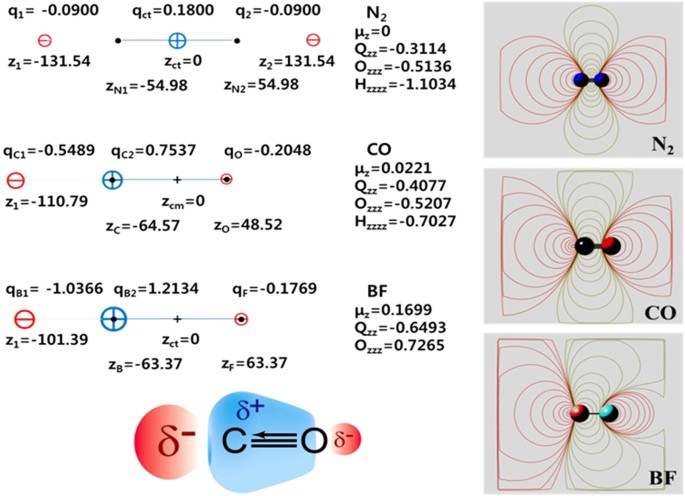
Intriguing Electrostatic Potential of CO: Negative Bond-ends and Positive Bond-cylindrical-surface | Scientific Reports
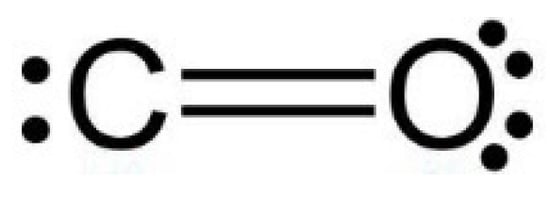
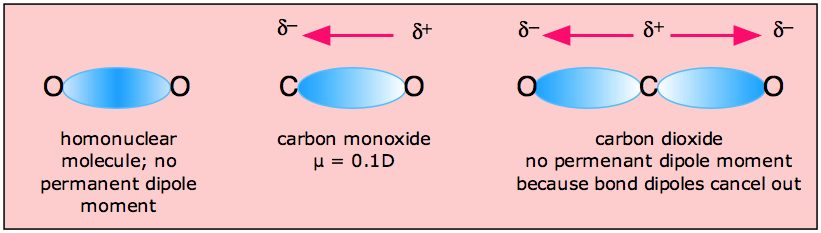
Minerals | Free Full-Text | CO2 Dipole Moment: A Simple Model and Its Implications for CO2-Rock Interactions
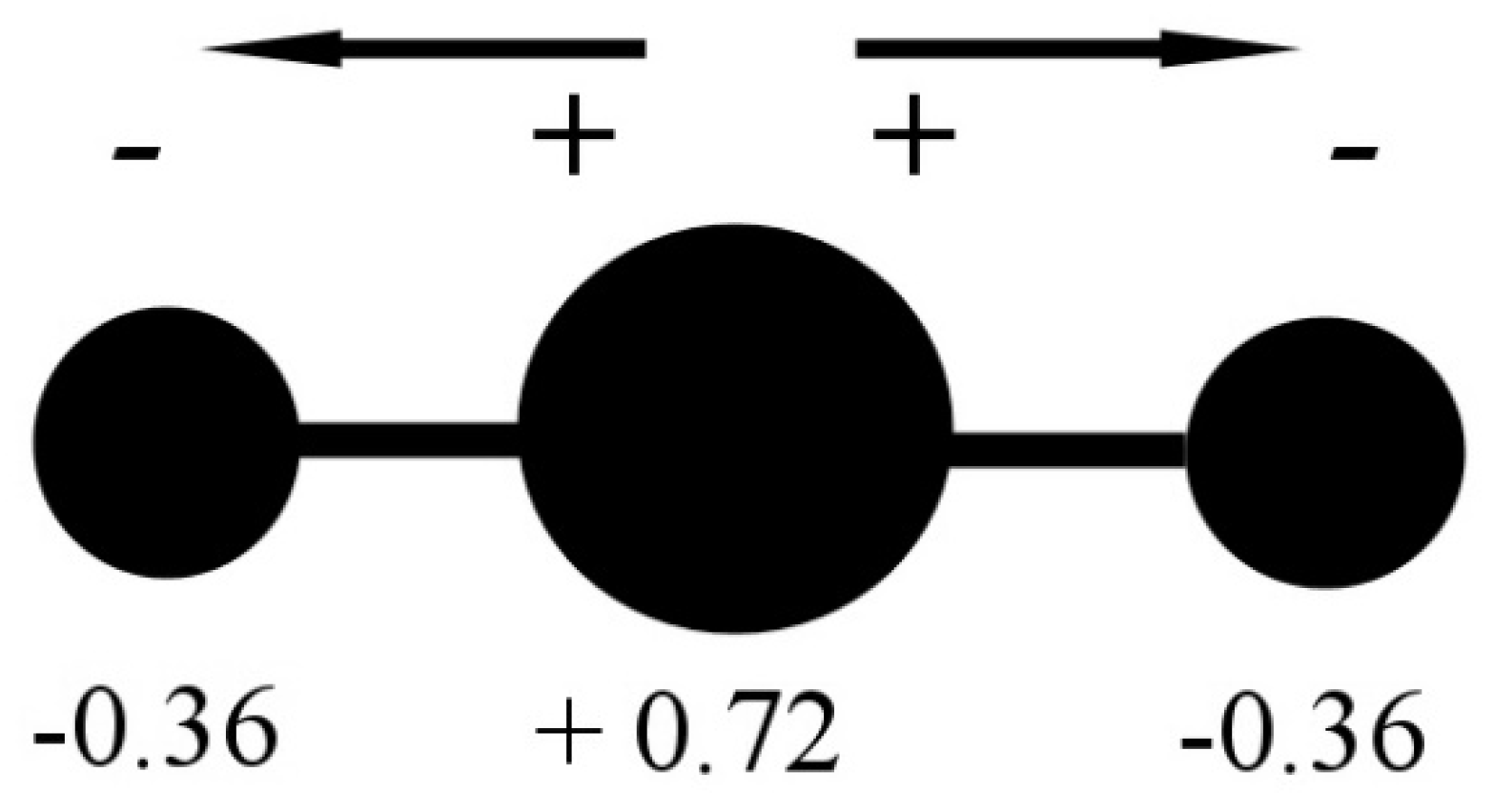
Minerals | Free Full-Text | CO2 Dipole Moment: A Simple Model and Its Implications for CO2-Rock Interactions

What are the most important types of interparticle forces present in the solids of carbon monoxide? | Homework.Study.com

Relationship between the Charge Distribution and Dipole Moment Functions of CO and the Related Molecules CS, SiO, and SiS | The Journal of Physical Chemistry A

Dipole moment functions of the CO molecule in the X 1 + and A 1 states.... | Download Scientific Diagram