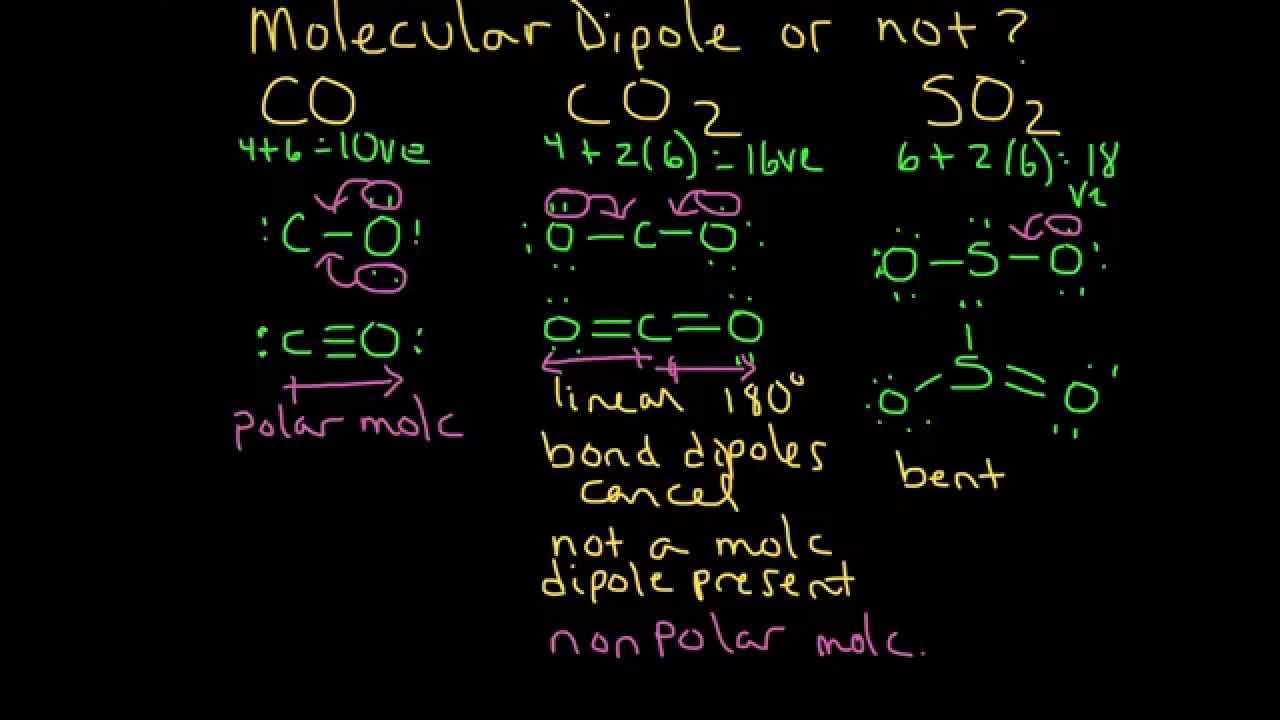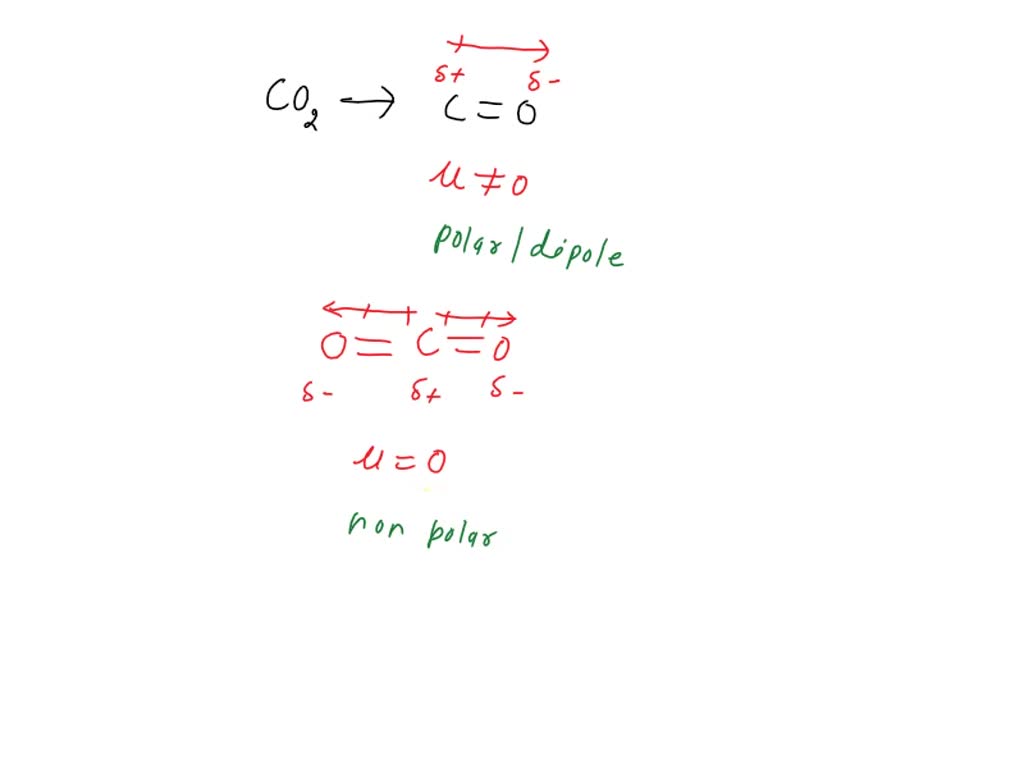
SOLVED: Polarity of bonds in CO2 C = O: Dipole Milh negative O Net dipole moment of CO2 0 = C = O; 0 = C = O; O = C =

Dipole moment of CO_{2} molecule is zero where as SO_{2} has some dipole moment. Explain the reason.

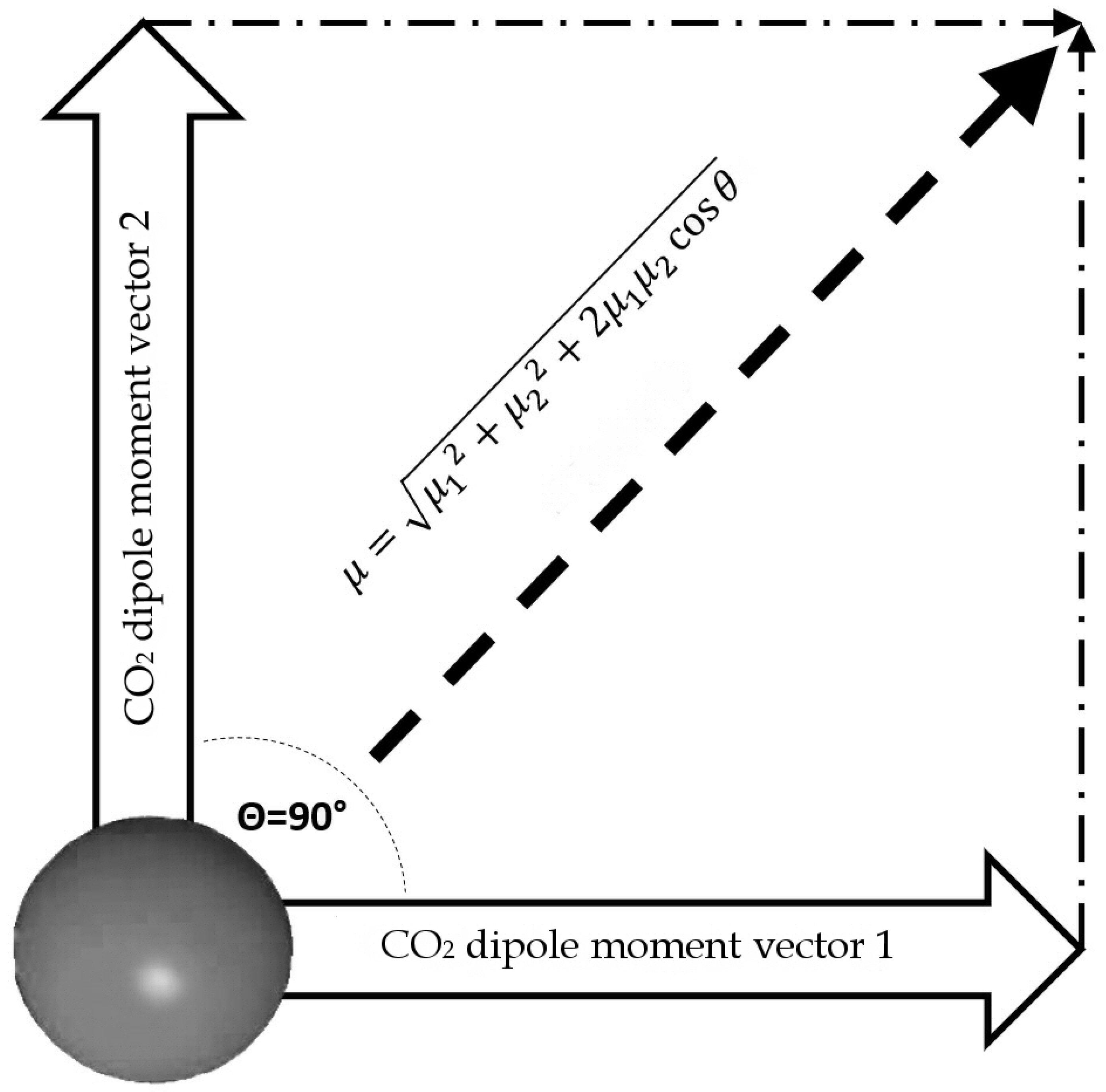
Minerals | Free Full-Text | CO2 Dipole Moment: A Simple Model and Its Implications for CO2-Rock Interactions
Define dipole moment? Comment on structure & dipole moment of CO2, BF3. - Sarthaks eConnect | Largest Online Education Community
Carbon dioxide has two polar bonds, but it is a non-polar molecule. Who can explain this further? - Quora
![The dipole moments of SO2 and CO2 are [tex]5.37 \times 10^{-30}[/tex] C.m and zero respectively. What can - Brainly.in The dipole moments of SO2 and CO2 are [tex]5.37 \times 10^{-30}[/tex] C.m and zero respectively. What can - Brainly.in](https://hi-static.z-dn.net/files/d7a/a0a7000831089a142800401faf38bc24.jpg)
The dipole moments of SO2 and CO2 are [tex]5.37 \times 10^{-30}[/tex] C.m and zero respectively. What can - Brainly.in
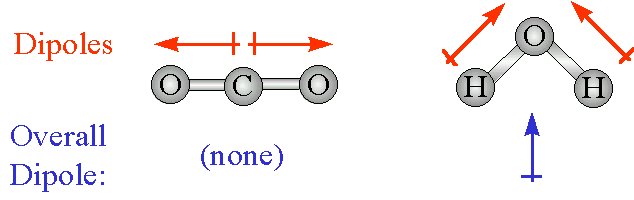
Which of the following compounds exhibits only dispersion and dipole-dipole intermolecular interactions ? A) HBr B) CO2 C) H2O D) N2 | Socratic
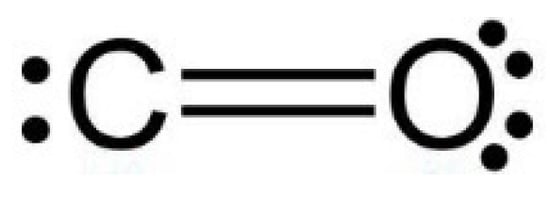
Minerals | Free Full-Text | CO2 Dipole Moment: A Simple Model and Its Implications for CO2-Rock Interactions

Draw the chemical structure for carbon dioxide. Show the bond dipoles and overall dipole moment. | Homework.Study.com