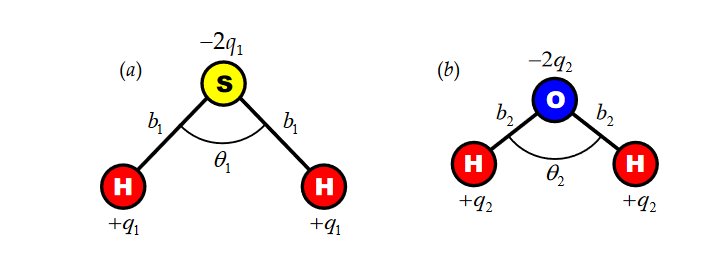
90 DUT8U J U TU Dipole moment of H2S is 0.95 D. Calculate the bond moment the bond angle is 97" (Cos 48.5° = 0.662) 0.7170 thi 1 275 Å The percentage of one

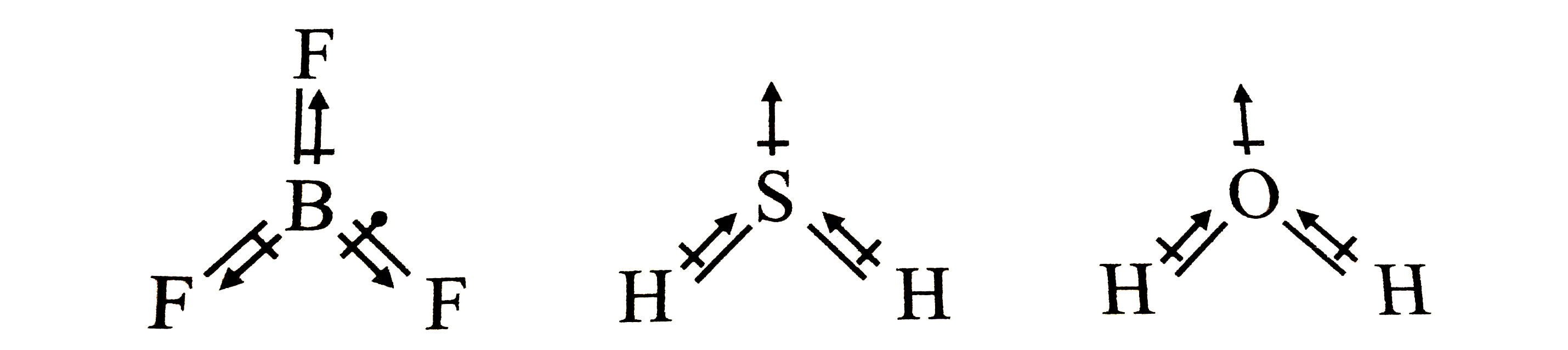
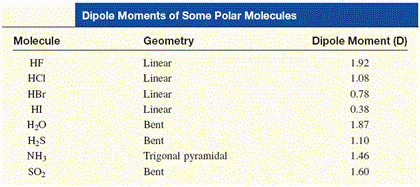
Arrange the following in order of increasing dipole moment H20,H2S,BF3 - Chemistry - Chemical Bonding and Molecular Structure - 12928599 | Meritnation.com
Explain the difference in the solubility of water and hydrogen sulfide in hexane? Solubility of water in hexane is 0.01 g/100g Solubility of hydrogen sulfide in hexane is 0.7g/100g? | Socratic

Calculations of dipole moments of H2S and PH3 - Transactions of the Faraday Society (RSC Publishing)

out of Co2 and H2S which pair has dipole moment - Chemistry - Chemical Bonding and Molecular Structure - 12896827 | Meritnation.com





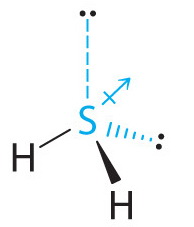





![Is [math]H_2S[/math] polar or nonpolar? - Quora Is [math]H_2S[/math] polar or nonpolar? - Quora](https://qph.cf2.quoracdn.net/main-qimg-60b021aa16478c549d60c016b6a83f1f.webp)